Ophthalmic Formulation Development: Inside Ophthalmic Preparation
Ophthalmic formulation development is receiving more attention in the pharmaceutical industry as of late thanks to advances in technology, increasing awareness of eye diseases, and an aging population. As people live longer, the need to find innovations in treating cataracts, macular degeneration, glaucoma, and diabetic retinopathy grows.
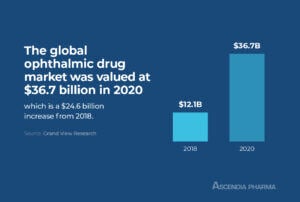
The global ophthalmic drug market was valued at $36.7 billion in 2020 and is expected to have a compound annual growth rate (CAGR) of 6.4 percent from 2021 to 2028. As pharmaceutical companies anticipate continued growth in this market, the demand for contract development and manufacturing organizations (CDMOs) with experience in the formulation of ophthalmic products is on the rise.>> Related Read – Top CDMO Companies: Why Bigger Isn’t Always Better
What Are Ophthalmic Preparations?
Ophthalmic preparations are drugs applied to the eye or surrounding tissues. Drugs for ocular administration come in many forms, including solutions, suspensions, gels, and ointments, and they can be used to deliver an active pharmaceutical ingredient (API) to the surface of the eye, inside the eye, adjacent to the eye, or they can be used with an ophthalmic device.
There are a number of ophthalmic preparations available over-the-counter for general sale, with most offering treatments for dry eye or redness. Prescription ophthalmic preparations address a wide range of issues, from glaucoma to bacterial or viral infections.
Types of Ophthalmic Preparations
The classification of ophthalmic drugs can be broadly divided into two categories: topical dosage forms and intraocular dosage forms. The following dosage forms are among the most well-known ophthalmic preparations:

Eye Drops
Eye drops are the most prescribed ocular dosage form thanks to their safety, efficacy, and ease of use. They have immediate effects, and because they’re non-invasive, patient compliance is high.
Despite this, traditional eye drop formulations do have some limitations. The membrane of the corneal tissue is lipophilic, which means it has a low affinity for the hydrophilic cyclodextrin molecules and other excipients used to encapsulate ophthalmic drugs in eye drop formulations. The cyclodextrins do not pass through the cell membrane and remain in the exterior aqueous membrane until they’re cleared away by lacrimal fluid, which has the potential to sensitize the eye, causing inflammation, irritation, and blurred vision.
To overcome this obstacle, emulsions, ointments, and suspensions were developed to improve bioavailability and solubility, and also increase the time the drug spends in the precorneal tissues.
Suspensions
Suspensions are dispersions made using a hydrophilic solvent. The particles in the suspicion are taken by the precorneal tissue, which extends the contact time of the drug with the tissues and thus increases the time in which the drug has therapeutic effects. The larger the particle size, the slower the dissolution and the longer the retention time will be.
>> Related Read – Types of Sterile Dosage Forms (and How to Choose)
Solutions
Ophthalmic solutions are sterile aqueous solutions that may or may not contain preservatives and excipients to regulate pH, viscosity, and osmotic pressure. They are primarily used for cleansing and rinsing the eye.
Microemulsions
Microemulsions are a newer drug form that offers increased bioavailability and the ability to introduce larger amounts of an API into the eye. They are stable, easy to sterilize, and inexpensive to produce. These drugs work through the adsorption of nanodrops that form a reservoir of the drug and an inner phase of microemulsion on the corneal surface.
Ointments
Ointments are a semisolid dosage form typically made with either a solid or semisolid hydrocarbon base that has a softening or melting point close to human body temperature. When the ointment is applied to the eye, it breaks into small drops. Ointments overcome bioavailability concerns because these droplets remain present in the conjunctival sac for an extended time. The downside of this is that they can cause blurring of vision and sometimes also irritation, so they are commonly prescribed for night time use.
Conjunctival Inserts
Conjunctival inserts are solid, semisolid, or gel-like ophthalmic formulations that are placed directly on the conjunctiva. They are a newer type of formulation and offer several advantages over other types of ocular drug delivery.
One benefit is that they provide a large surface area for drug absorption, which leads to more consistent and reliable drug delivery. They also have a longer retention time in the eye, which can extend the therapeutic effects of the drug. The downside of conjunctival inserts is that they can be difficult to insert and remove, and may cause irritation or discomfort.

Intraocular Injections
Intraocular injections are used to deliver a therapeutic dose of a medication directly into the eye. This allows for a very high concentration of a drug to be delivered directly where it is needed, which can be useful for treating many eye conditions. Of all the ocular drug delivery forms, though, intraocular injections are among the least desirable for patients, as they are invasive and can cause discomfort or pain.
>> Related Read – Sterile Injectable Drugs Defined
Contact Lenses
Contact lenses can be coated with drugs and used as an ocular delivery system. The contact lenses absorb water-soluble substances on its surface, then these are released when applied to the eye. This system can provide a more consistent and controlled drug release over time.
Gels
Gels are semisolid formulations that are typically made from hydrophilic polymers. They can be used to deliver both water-soluble and insoluble drugs to the eye. The advantage of using gels is that they can provide a controlled release of the drug over time, but they may cause irritation or discomfort when applied to the eye.
Mucoadhesive Polymers
Mucoadhesive polymers are a type of formulation that is being developed for ocular drug delivery. They are designed to adhere to the mucous membranes in the eye and provide a controlled release of the drug over time. Mucoadhesive polymers are being explored as a method of nano-drug delivery.
Implants
Intraocular implants deliver a drug cocktail to a targeted location during a specific time range, preventing the need for multiple injections into the ocular tissues, which cause inflammation and have the potential for infection. When a drug must be delivered to the back of the eye, a minor surgical procedure is required to implant the device.
Nanoparticles
Nanoparticles are a newer type of formulation that is being developed for ocular drug delivery. They offer the potential for increased bioavailability and the ability to target specific tissues in the eye, as their small size means they are better able to penetrate the ocular surface epithelium, tear film, and blood-aqueous and blood-retinal barriers. Nanoformulations also hold potential to treat retinal disease through delivering nucleic acids for gene transfer therapy.
The Challenges of Ophthalmic Preparations
The eye is unlike any other organ in the body. It has a unique structure, with a complex anatomy designed to protect itself from harm. The natural protective barriers of the eye present a challenge in ophthalmic formulation development; any drug delivered ocularly must be able to bypass the multiple layers of the eye surface in order to do its job.
In other words, the eye is specifically designed — anatomically and chemically — to prevent the penetration of foreign substances. This creates a problem of low bioavailability for many drugs.
It’s also critical that ophthalmic preparations are sterile, as they come into direct contact with the cornea and precorneal tissue. These tissues can absorb bacteria and other contaminants, with the potential for serious health risks.
To improve bioavailability, ophthalmic formulations need to have a certain viscosity that enables them to stay in contact with the cornea for an extended amount of time. This is achieved by adding hydrophilic polymers with high molecular weight, as they do not diffuse through biological membranes.
Finally, ocular drugs must have a pH level equal or as close as possible to tear fluid. Most drugs are unstable at this pH, so a buffer must be added, but this, too, must be carefully considered, as the added buffer can also cause chemical instability.
>> Related Read – Formulation Development: Why It’s So Important

Ophthalmic Formulation Evaluation
A successful ophthalmic formulation must meet several criteria. First and foremost, it needs to be safe and effective. It should also be stable in storage and shipment, with a long shelf life, and, ideally, its form will be desirable and tolerable to patients.
In terms of safety, ophthalmic preparations are parenteral products, and as such, they need to be free of all microorganisms; all ocular products must undergo a sterility test to ensure that they contain no pyrogens. Next, a clarity test verifies that there are no undissolved ingredients or foreign particles; ointments are tested for metal particles, and packaging is tested for leaks.
After in vitro and in vivo testing, a formulation is ready for clinical trials. Clinical trials for ophthalmic preparations are no different than those for other drugs, and they are conducted in three phases. Phase I trials assess the safety of the formulation in humans. Phase II trials assess the efficacy of the formulation in humans. Phase III trials confirm the safety and efficacy of the formulation in humans.
After clinical trials, the formulation is submitted to the FDA for approval. From here, production is scaled up to bring the drug to market.
Partner with Ascendia for Ophthalmic Formulation and Development
Ascendia specializes in innovative topical formulations, including nano-emulsions that are suitable for ophthalmic drop application to the eye. We offer dosage form development for poorly soluble and low bioavailability drugs, and we’re leading the way in the development of nanopharmaceuticals.
Learn more about partnering with Ascendia, and contact us today.

