Streamlined Development
In the intricate landscape of biopharmaceuticals, development timing is a critical factor that can significantly impact the success or failure of a drug. Saving time can mean the difference between being first to market for a new drug class or a first-to-file generic.
The journey from initial discovery to market availability in biopharma requires meticulous orchestration. This process is typically fraught with challenges, uncertainties, and substantial investments.
Understanding and optimizing development timing is paramount as it directly influences various facets of drug development, regulatory approvals, market competitiveness, and patient access.
Biopharma: No Shortage of Competition
One of the primary reasons development timing is crucial in biopharma lies in the competitive nature of the industry.
The well-known characteristics of the biopharmaceutical market are fierce competition, rapid advancements in technology, and evolving regulatory frameworks. Companies must navigate a complex maze of clinical trials, regulatory submissions, and intellectual property considerations.
Somehow, they must manage all that while striving to bring their therapies to market ahead of competitors. Timing can make all the difference between being the first mover with groundbreaking treatment and being left behind in a crowded field.
Strategic Timing and the Bottom Line
Development timing profoundly impacts the financial aspects of biopharmaceutical endeavors. The journey from preclinical research to commercialization demands substantial financial resources. Any delays at any stage can escalate costs significantly.
Time is money in biopharma, and prolonged development cycles drain resources and delay potential revenue streams.
Investors, stakeholders, and shareholders closely monitor timelines. Missed deadlines or extended development periods can erode confidence and impede future funding prospects.
Project Timing and Public Health Considerations
In addition to financial considerations, development timing directly affects patient outcomes and public health. Every day lost in the development process translates to delayed access to potentially life-saving treatments for patients eagerly awaiting new therapies.
In diseases where time is of the essence — such as certain cancers or rare genetic disorders — delays in drug development can have profound implications for patient well-being and survival rates. Optimizing development timing is a matter of commercial success, but it also carries a moral imperative to address unmet medical needs and improve healthcare outcomes.
As the biopharmaceutical industry evolves, stakeholders must remain vigilant in managing development timelines efficiently, leveraging innovative approaches, and navigating regulatory landscapes to expedite the journey from bench to bedside.
Engaging the Experiences and Expertise of a CDMO
Contract Development and Manufacturing Organizations (CDMO) such as Ascendia Pharmaceutical Solutions play a pivotal role in assisting drug development projects to establish and adhere to timelines for delivering a project to clinical trial.
Saving time during every stage of the drug development process is a critical factor. CDMOs are vital in supporting drug development projects by providing expertise, resources, and infrastructure to develop and maintain timelines for delivering projects to clinical trials.
CDMOs provide services that streamline the drug development process, optimize resource allocation, and ensure efficient project management. Here are several ways by which a CDMO such as Ascendia can assist in developing and maintaining a timeline for clinical trial delivery with CROs:
Efficient Site Selection and Patient Recruitment
Streamlined Protocol Development
Project Management and Oversight
Data Management and Analysis
Risk Management and Mitigation
Through strategic planning, efficient execution, and proactive risk management, CDMOs help sponsors navigate the complexities of the drug development process and accelerate the path to regulatory approval and market launch.
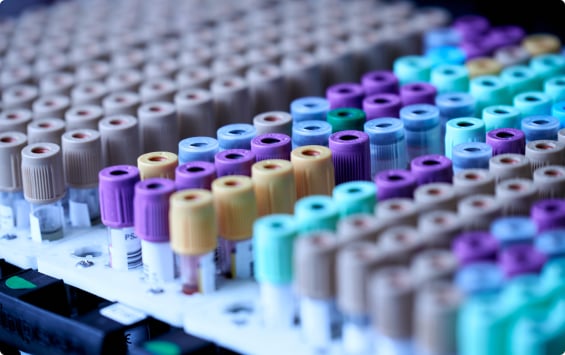
Optional Eyebrow Headline
Eyebrow Text
Body text to go here lorem ipsum dolor sit amet consectetur adipiscing elit. Text link example amet pharetra rhoncus bibendum dui amet, tristique ut arcu fringilla. Lorem ipsum dolor sit amet, consectetur adipiscing elit bibendum dui.
Consider the Ascendia Advantage
The industry-seasoned team at Ascendia fully recognizes the value of rapid development timelines. We offer services designed to quickly reach early-stage key milestones in your development program.
Our services include pre-formulation, enabling formulations for toxicology and pharmacokinetic studies, formulation scale up, and early -phase and late stage clinical trial materials for oral or parenteral products.
Partnering with Ascendia Pharma means gaining access to expertise, innovative solutions, and strategic insights that can transform your development trajectory. Ensure project development timing concerns are sufficient for your progress and maintain your competitive edge. Take action today and unlock the full potential of your drug development initiatives with Ascendia Pharma by your side.
Reach Out Today
If your company is facing challenges with project development timing, look to Ascendia for expert solutions tailored to your needs.
With our proven track record of success and unwavering commitment to excellence, Ascendia offers a comprehensive suite of services to streamline your drug development timelines. We help propel your projects toward success, whether you need help with regulatory hurdles, patient recruitment delays, or resource constraints. Our seasoned professionals are ready to assist you every step of the way.
Contact us now to learn more about how our tailored solutions can help you overcome obstacles, meet critical milestones, and bring life-changing therapies to market faster.